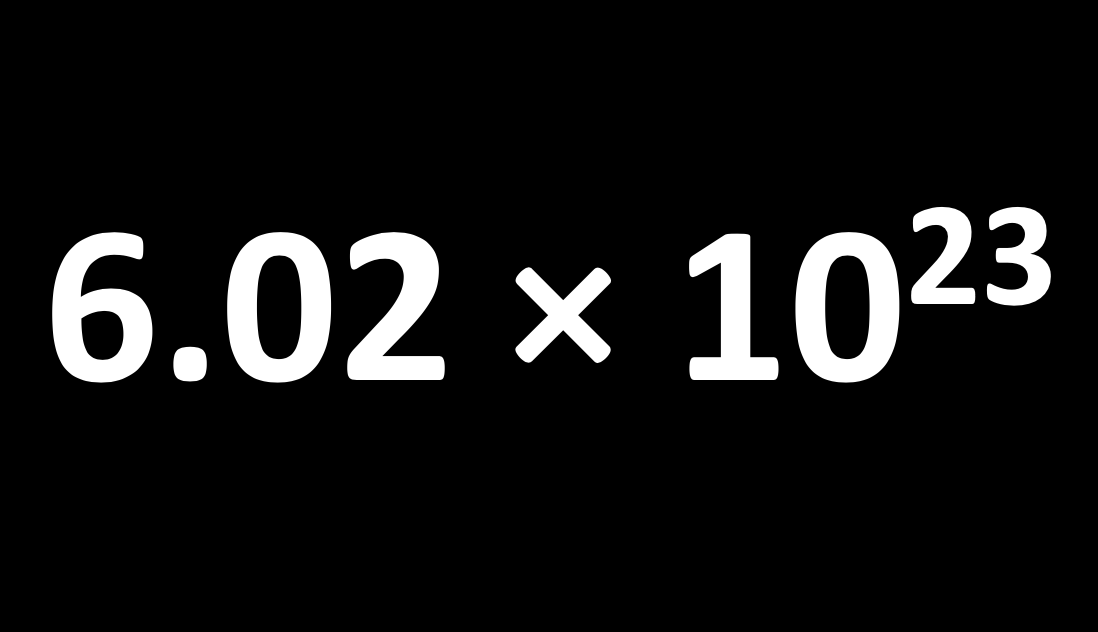 A mole is simply a number, specifically Avogadro's number (L) which is 6.02 ×1023. It is the number of atoms in exactly 12 g of pure carbon-12.
A mole is simply a number, specifically Avogadro's number (L) which is 6.02 ×1023. It is the number of atoms in exactly 12 g of pure carbon-12.
Mole calculations are not difficult in terms of the mathematical functions that you need to carry out, but it can be difficult to work out what needs to be added, divided, multiplied etc. Use revision card 4 to review how the the empirical formula of a compound can be experimentally determined. Then use the practice questions to ensure you are confident calculating the formulae from raw data.
Ensure you are confident using the terms below and learn the asterisked* definitions
Avogadro constant (L)*, molar mass (M), mole (n)*, empirical formula, molecular formula, percentage by mass
What is the relative molecular mass (Mr) of magnesium nitrate, Mg(NO3)2?
To find the Mr the relative atomic masses of all the atoms are added together. Remember to use the values from the data book, given to two decimal places:
24.31+((14.01+16.00×3)×2) = 24.31+124.02 = 148.33
What is the molar mass of carbon, and its units?
The molar mass is the mass of one mole, and therefore has units of grams per mole (g mol−1 or g/mol).
Relative atomic/molecular/formula masses have no units (as they are relative) but molar mass does have units.
Remember to use the relative atomic mass values from the data book, given to two decimal places when calculating the molar mass.
How many atoms of hydrogen are present in one mole of ethane, C2H6?
One mole of ethane is 6.02×1023 molecules of ethane (L, Avogadro's constant = 6.02×1023).
Each molecule has six hydrogen atoms in it. So there will be 6 × 6.02×1023 atoms of hygrogen.
6 × 6.02×1023 = 36.12×1023 = 3.61×1024 given to 3 sig figs.
How many chloride ions will be released into water when 0.250 mol of calcium chloride (CaCl2) is dissolved completely in water?
One mole of calcium chloride is 6.02×1023 'formula units' of CaCl2 (L, Avogadro's constant = 6.02×1023).
Each 'formula unit' will release two chloride ions: CaCl2 → Ca2+ + 2Cl−
So, 0.250 moles of calcium chloride, will release 0.500 moles of chloride ions:
0.500 × 6.02×1023 = 3.01×1023 given to 3 sig figs.
To two significant figures, how many atoms of oxygen are present in 640g of oxygen?
One mole of oxygen atoms is 6.02×1023 atoms (L, Avogadro's constant = 6.02×1023).
Moles = mass/molar mass, so 640/16.00 = 40 mol
40 moles of oxygen atoms will be 40 × 6.02×1023 atoms of oxygen.
40 × 6.02×1023 = 240.8×1023 = 2.408×1025
Thus 2.4×1025 is the correct answer.
A compound has an empirical formula of CH, and a molecular mass of approximately 104. What is the molecular formula of the compound?
The empirical formula is the simplest ratio of atoms in the molecule/formula. The molecular formula represents the actual number of atoms present in the molecule.
The empirical 'formula unit' has a mass of approx. 13 (12+1), and the molecular mass of the molecule is 104.
104 / 13 = 8
So there are eight 'formula units' of the empirical formula in the molecule: The molecular formula is C8H8.
A sample of a pure compound from a vanilla plant is analysed and shown to consist of 48.04g of carbon, 4.04g of hydrogen and 24.00g of oxygen by mass. What is the empirical formula of the compound?
Find the simplest ratio of the number of (moles of) atoms.
| Carbon | Hydrogen | Oxygen | |
moles = mass/molar mass | 48.04/12.01 = 4 | 4.04/1.01 = 4 | 24.00/16.00 = 1.5 |
| multiply up to whole numbers (×2) | 8 | 8 | 3 |
Thus the answer is C8H8O3.
A compound consists of 53% carbon, 6% hydrogen and 41% nitrogen by mass. What is the empirical formula of the compound?
Find the simplest ratio of the number of (moles of) atoms.
Start by assuming 100g: Thus 53g of C atoms, 6g of H atoms, 41g of N atoms.
| Carbon | Hydrogen | Nitrogen | |
moles = mass/molar mass | 53/12.01 = 4.412989176 | 6/1.01 = 5.940594059 | 41/14.01 = 2.926481085 |
divide through by smallest | 4.41../2.92.. ≈ 1.5 | 5.94../2.92.. ≈ 2 | 2.92../2.92.. = 1 |
| multiply up to whole numbers (×2) | 3 | 4 | 2 |
Thus the answer is C3H4N2.
When phosphorus reacts with chlorine a yellow liquid is formed.
12.39g of phosphorus reacted completely to form 54.93g of the phosphorus chloride.
What is the empirical formula of the phosphorus chloride?
Find the simplest ratio of the number of (moles of) atoms.
Start by finding the mass of chlorine that reacted:
Mass of chlorine = mass of phosphorus chloride minus mass of phosphorus = 54.93 − 12.39 = 42.54g
| Phosphorus | Chlorine | |
moles = mass/molar mass | 12.39/30.97 = 0.400064578 | 42.54/35.45 = 1.2 |
divide through by smallest | 0.400../0.400.. = 1 | 1.2 / 0.400.. ≈ 3 |
| whole numbers | 1 | 3 |
Thus the answer is PCl3.
A carbohydrate consists of 40.0% carbon, 6.7% hydrogen and 53.3% oxygen by mass, and has a molar mass of approximately 120 g mol−1.
What is the molecular formula of the compound?
Find the simplest ratio of the number of (moles of) atoms.
Start by assuming 100g: Thus 40.0g of C atoms, 6.7g of H atoms, 53.3g of O atoms.
| Carbon | Hydrogen | Oxygen | |
moles = mass/molar mass | 40.0/12.01 = 3.330557868 | 6.7/1.01 = 6.633663366 | 53.3/16.00 = 3.33125 |
divide through by smallest | 3.33../3.33.. = 1 | 6.63../3.33.. ≈ 2 | 3.33../3.33.. ≈ 1 |
| whole numbers | 1 | 2 | 1 |
Thus the empirical formula is CH2O.
The empirical 'formula unit' has a mass of approx. 30 (12+2+16), and the molecular mass of the molecule is approx. 120.
120 / 30 = 4
So there are four 'formula units' of the empirical formula in the molecule: The molecular formula is C4H8O4.













 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn