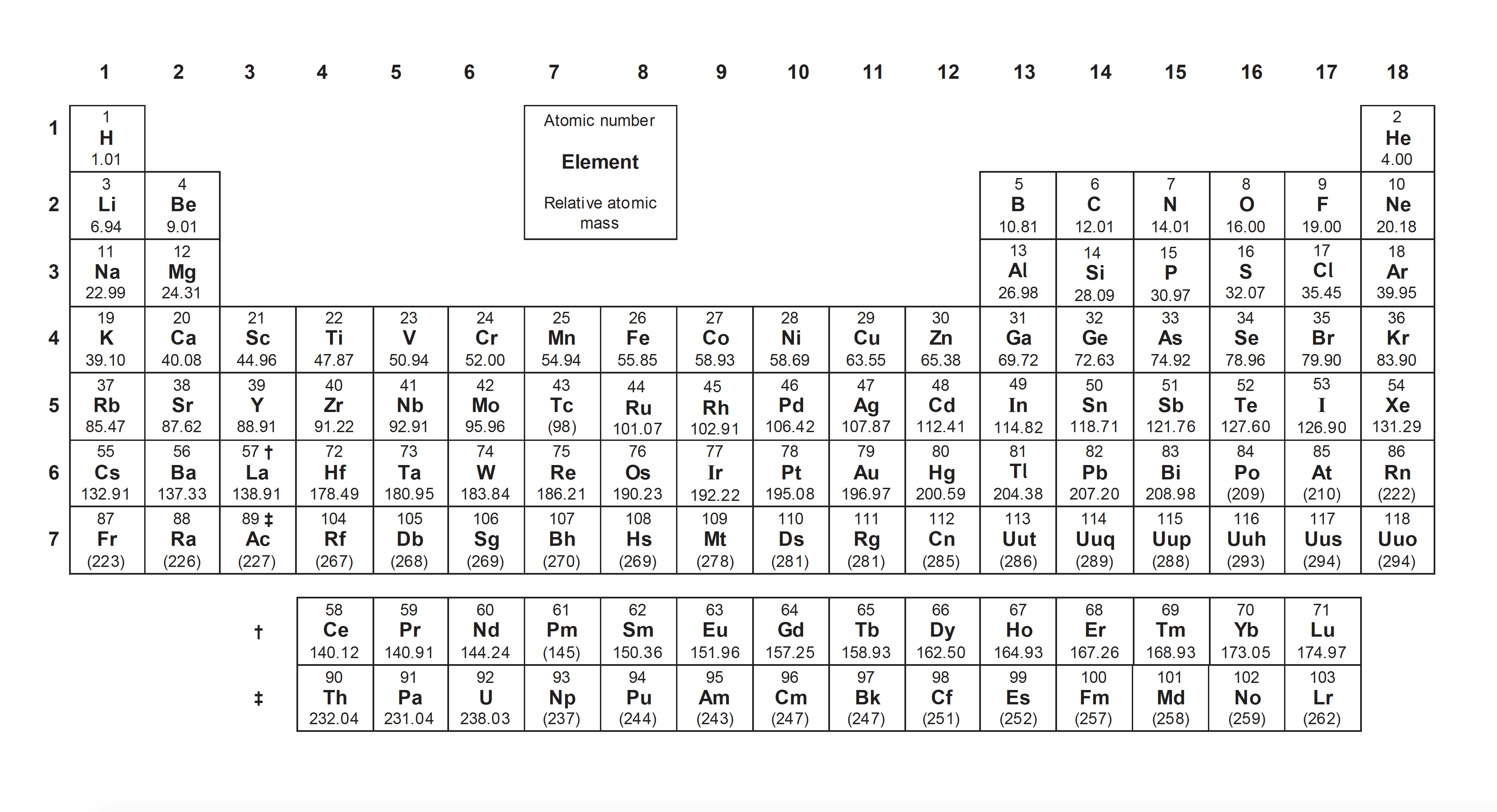
 Periodicity examines the trends in chemical and physcial properties across and down the periodic table of elements. The trends are 'periodic', in that they repeat themselves. The periodic table of elements is arranged in order of proton number, and in accordance with our quantum mechanical model of the electronic structure of atoms.
Periodicity examines the trends in chemical and physcial properties across and down the periodic table of elements. The trends are 'periodic', in that they repeat themselves. The periodic table of elements is arranged in order of proton number, and in accordance with our quantum mechanical model of the electronic structure of atoms.

Coloured complexes
Topic 13.2 Exploring how colour arises in transition metal compounds.

First-row d-block elements
Topic 13.1 Introducing the d-block elements.

Periodic table
Topic 3.1 The periodic table basics.

Periodic trends
Topic 3.2 A big section summarising many trends on the periodic table.

 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn