 Topic 2: Molecular biology
Topic 2: Molecular biology
This is an introduction to the molecular biology topic. It lists understandings and skills expected for Topic 2 including water, carbohydrates, lipids, proteins, enzymes, DNA, RNA, protein synthesis, respiration and photosynthesis. Helpful for revision.
Detailed revision notes, activities and questions can be found for each of the sub-topic pages:
- 2.1 Molecules to metabolism.2
- 2.2 Water
- 2.3 Carbohydrates and lipids
- 2.4 Proteins
- 2.5 Enzymes
- 2.6 Structure of DNA and RNA
- 2.7 DNA replication, transcription and translation
- 2.8 Cell respiration
- 2.9 Photosynthesis
2.1 Molecules to metabolism
Carbon based compounds
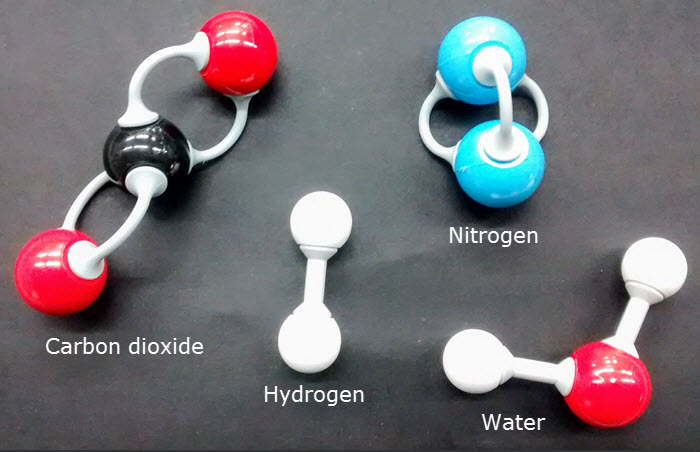
- Molecular biology is explaining biological processes in terms of the chemicals involved.
- The is a diversity of Carbon based compounds in living things because carbon atoms can form four covalent bonds.
e.g. carbohydrates, lipids, proteins & nucleic acids. - All the enzyme-catalysed reactions in a cell make up its metabolism. There are two types:
- Anabolism: forming macromolecules from monomers by condensation.
- Catabolism: breaking complex macromolecules into simpler molecules by hydrolysis.
- Some biological compounds can be synthesized outside of living things: e.g. urea.
Skills
- Draw diagrams of:
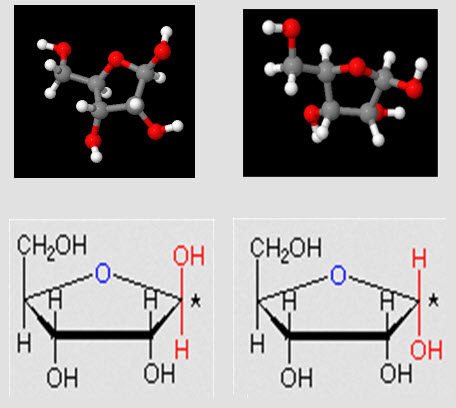
- αD-glucose & βD-glucose,
- D-ribose,
- a fatty acid
- an amino acid with generalised R-group.
- Identification of biochemicals from diagrams to include:
- monosaccharides
- disaccharides,
- lipids (triglycerides, phospholipids and steroids)
- amino acids
- polypeptides and peptide bonds.
- Experience of the Benedicts reagent test for reducing sugars and iodine to test for starch.
2.2 Water
- Hydrogen bonds form between polar water molecules.
- This force give water special properties, e.g. cohesiveness, adhesiveness, thermal
and solvent properties. - Glucose, amino acids and salts are hydrophilic while cholesterol and fats are hydrophobic.
Skills
- Compare the thermal properties of water with those of methane and explain how this affects its use as a coolant in sweat.
- Compare the solubility of glucose, amino acids, cholesterol, fats, oxygen and sodium chloride in water and link this to the way they are transported.
- Explain benefits of water properties to living organisms (Transparency and density not required.)
- Evaluate how significant hydrogen bonding is in the properties of water
2.3 Carbohydrates & Lipids
- Condensation reactions link Monosaccharide monomers together to
form disaccharides (Sucrose, lactose and maltose) and polysaccharides.(cellulose starch and glycogen) - Fatty acids can be saturated, monounsaturated or polyunsaturated.
and cis or trans isomers if they are unsaturated. (molecule names not required) - Three fatty acids and one glycerol molecules can form a Triglycerides by condensation reactions.
- How the structure of cellulose and starch (amylose & amylopectin) in plants and glycogen in humans relates to function.
- For long-term energy storage in humans
lipids are better than carbohydrates. - Potatoes have been genetically modified to reduce the level of amylose to
produce a more effective adhesive.
Skills
- Evaluate the conflicting evidence for health risks of trans fats and saturated fatty acids and evaluate the methods used
- Abiliy to use molecular visualization software like jmol to compare cellulose, starch and glycogen.
- Ability to work out a BMI using a nomogram or by calculation
2.4 Proteins
- Amino acids are linked together by condensation reactions to form a di-peptides and polypeptides.
- Genes and mRNA strands code for 20 different amino acids which are built into polypeptides on ribosomes.
- Amino acids can be linked together in any sequence (coded for by genes) giving a huge range of possible polypeptides.
- A protein may be a single polypeptide or more than one polypeptide joined together.
- The three-dimensional shape of a protein is determined by the sequence of amino acids.
- Living organisms synthesize many different proteins with a wide range of functions (not structure)
e.g. Rubisco, insulin, immunoglobulins, rhodopsin, collagen & spider silk. - Every individual has a unique proteome.
- Proteomics and the production of proteins by cells cultured in fermenters
offer many opportunities for the food, pharmaceutical and other industries
(a utilisation)
Skills
- Ability to draw molecular diagrams of peptide bond formation.
2.5 Enzymes
- The role of the active site where specific substrates bind.
- The effect of the motion of molecules and the collision of substrates with the active site.
- The effect of Temperature, pH and substrate concentration on the rate of activity of enzymes. (including denaturing)
- Enzymes (often immobilized) are extensively used in industry for the production of items including Lactose-free milk, fruit juice and washing powder.
Skills
- Advantages of latose-free milk, and ways of producing it, including immobilization in alginate beads.
- Knowledge of possible designs of experiments to test the effect of temperature, pH and substrate concentration on enzyme activity.
- Practical 3: Investigation of a factor affecting enzyme activity.
- The skill of sketching a graph of expected results in enzymes experiments and the ability to explain reasons for their shapes.
2.6 Structure of DNA and RNA
- DNA and RNA are polymers each made of nucleotides.
- A DNA nucleotide is made from phosphate, deoxyribose (pentose sugar), and nucleotide bases (T, A,G,C)
- RNA nucleotides are made from phosphate, ribose (pentose sugar) and nucleotide bases (U, A, G, C)
- Base pairing is "complementary"
- DNA is a double helix made of two strands of nucleotides linked by hydrogen bonding RNA is a single strand of nucleotides.
Skills
- Students should be able to draw simple diagrams of DNA and RNA nucleotides - using simple shapes (not chemical symbols)
- Students should learn to draw two antiparallel strands of DNA showing base pairing, A 'ladder' structure is enough, and details of purines / pyrimidines are not required
2.7 DNA Replication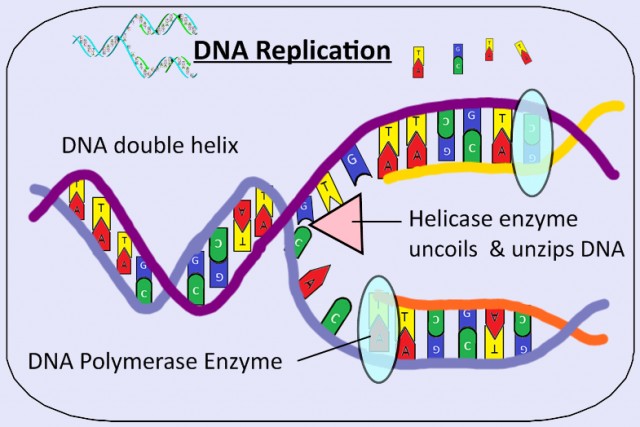
- DNA replication
- Complementary base pairing leads to the semi-conservative replication of DNA.
- The enzyme helicase unwinds the double helix and breaks hydrogen bonds which hold the two DNA strands together.
- DNA polymerase (generalised name) links DNA nucleotides together to form a new strand DNA,using the pre-existing strand as a template.
- Transcription is the synthesis of mRNA by RNA polymerase using the DNA base sequence as a template
- Translation is the synthesis of polypeptides on ribosomes.
- The amino acid sequence of polypeptides is determined by mRNA according to the genetic code.
- Three bases of mRNA is called a codon and corresponds to one amino acid in the polypeptide.
- Translation depends on complementary base pairing between codons on mRNA and anticodons on tRNA.
- Awareness that in polymerase chain reaction (PCR) an enzymes called Taq DNA polymerase produces multiple copies of DNA.
Skills
- Ability to explain how Meselson and Stahl’s results support for the theory of semi-conservative replication of DNA.
- knowledge that human insulin can be produced in bacteria because of the "universality" of the genetic code. This allows genes to be transferred between species.
- Ability to use a table of the genetic code to deduce which codon(s) corresponds to which amino acid and to deduce the sequence of amino acids coded by a short mRNA or the DNA base sequence for a given mRNA strand
2.8 Respiration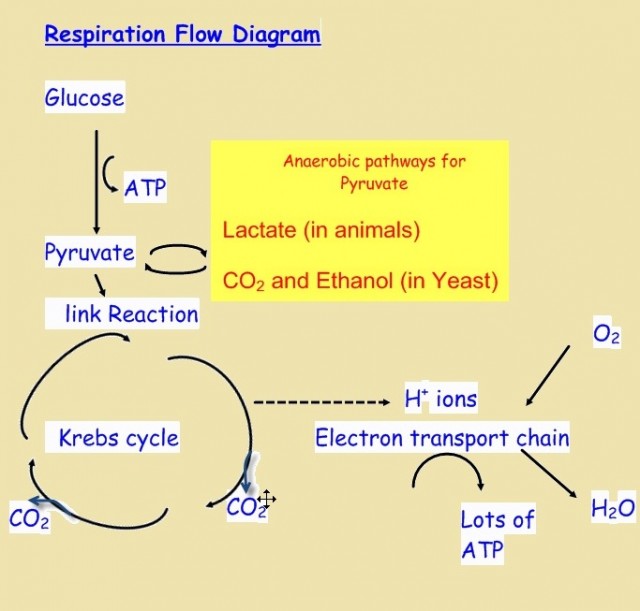
- Cell respiration definition,"the controlled release of energy from organic compounds to produce ATP"
- ATP produced is a source of energy ready for immediate use in the cell.
- Anaerobic cell respiration gives a small yield of ATP from glucose compared to aerobic respiration whose yield is large.
- Aerobic cell respiration also requires oxygen.
- Substrates (e.g. glucose) and final waste products (e.g. water, CO2, lactate, ethanol) should be known.
- Anaerobic cell respiration in yeasts is used to produce ethanol and carbon dioxide in baking.
- In the human body anaerobic respiration is used to maximize the power of muscle contractions & produces lactate.
Skills
- Know how to use simple respirometers to measure the rate of respiration.
- to know that an alkali is used to absorb CO2 produced in respirometers, so that reductions in gas volume are due to oxygen use.
- to keep the temperature constant, so that gas volumes don't change through expansion / contraction of gas.
2.9 Photosynthesis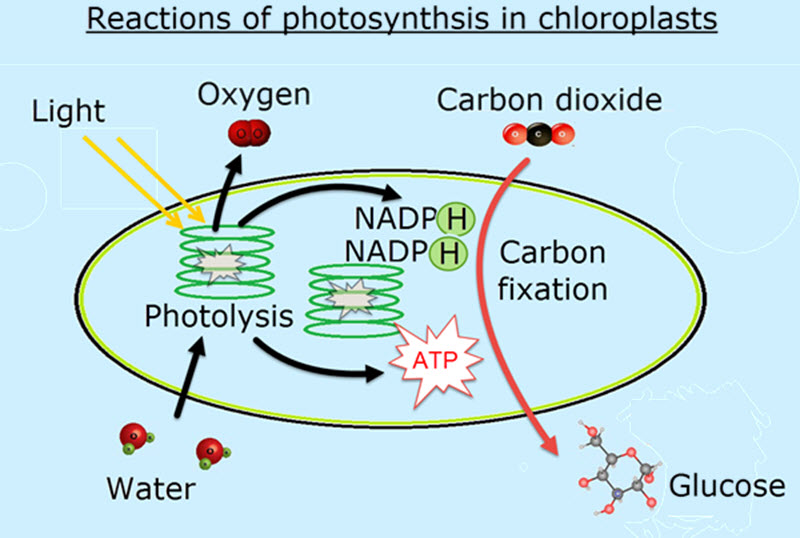
- Photosynthesis uses light energy to produce carbon compounds in cells.
- Visible light ranges from violet (400nm) the shortest wavelength to red (700nm) the longest.
(Students are not expected to recall the wavelengths of other colours.) - Absorption spectrum shows - red and blue light absorbed most and green light least (it is reflected).
- Photolysis of water produces oxygen
(and also ATP & NADPH) - Energy ( from photolysis) is needed to produce carbohydrates and other carbon compounds from carbon dioxide.
- Limiting factors of photosynthesis can be; Temperature, light intensity and carbon dioxide concentration.
- Understand that photosynthesis have caused changes to the Earth’s atmosphere, oceans and rock deposition.
Skills
- Learn how to draw
- an absorption spectrum for chlorophyll and
- an action spectrum for photosynthesis.
- Design experiments to investigate the effect of limiting factors on photosynthesis.
Ref: control of variables is essential. - Separate photosynthetic pigments by chromatography. (Practical 4) either by paper chromatography or thin layer chromatography (gives better results)

Molecules to metabolism 2.1
The structure of living organisms can be partly explained by the molecules which they are made from. Live is based on carbon because the way in which carbon atoms form covalent bonds is central to the structure of the molecules which make up organisms.
.png)
Water 2.2
In this section you will learn everything about the water, about its composition, polarity, hydrogen bonds and what makes water so unique in terms of its physical properties.

Carbohydrates & Lipids 2.3
This is a topic about three types of carbohydrates, monosaccharides, disaccharides and polysaccharides as well as lipids such as triglycerides which make up many oils in the human body. It includes the analysis of molecule shapes and their functions.

Proteins 2.4
This topic is all about proteins which are the most diverse of molecules. They are built from just twenty amino acids and show a huge variety of forms, from spider's silk to insulin hormones.

Enzymes 2.5
Enzymes control almost everything which happens inside the cells of all living organisms. In this topic we consider how enzymes make reactions faster as well as the effects of different environmental factors on the rate of enzyme controlled reactions.

Structure of DNA and RNA 2.6
In this topic a knowledge of the structure of nucleotides of DNA and RNA is required, as is a simple understanding of the structure of the double stranded, double helix, DNA.

DNA Replication, transcription, translation 2.7
This topic looks at these methods, DNA replication making an exact copy of the genetic material as well as Transcription and translation in the expression of a gene to make a protein.

Respiration 2.8
This topic covers the basics of aerobic and anaerobic respiration. It is important to know what the role of ATP is in cells and also to have experience of using a respirometer to measure the rate of respiration.

Photosynthesis 2.9
Photosynthesis is introduced in this topic. The main new idea is that the process is not a single step reaction but a sequence of reactions which occur in different parts of the chloroplast.

 Twitter
Twitter  Facebook
Facebook  LinkedIn
LinkedIn