The units of concentration, moles per decimetre cubed (mol dm−3), tells us the concentration equation:
Concentration (mol dm−3) = moles (mol) / volume of solution (dm3)
This can be rearranged:
Moles (mol) = concentration (mol dm−3) × volume of solution (dm3)
Volume of solution (dm3) = moles (mol) / concentration (mol dm−3)
1 dm3 = 1000 cm3 = 1 litre = 1000ml
Concentration can also be expressed in grammes per decimetre cubed (g dm−3) too, in which case moles must be converted to grammes.
Concentration of solution is the measure of the quantity of solute in a certain volume of solution (not volume of solvent). In chemistry, concentration is typically measured in moles per decimetre cubed (mol dm−3). Decimetres are not the SI (systeme internationale) unit of volume (that is metre cubed), but correspond to a litre, 1000cm3 or 1000ml, which is a sensible volume for use in the laboratory.
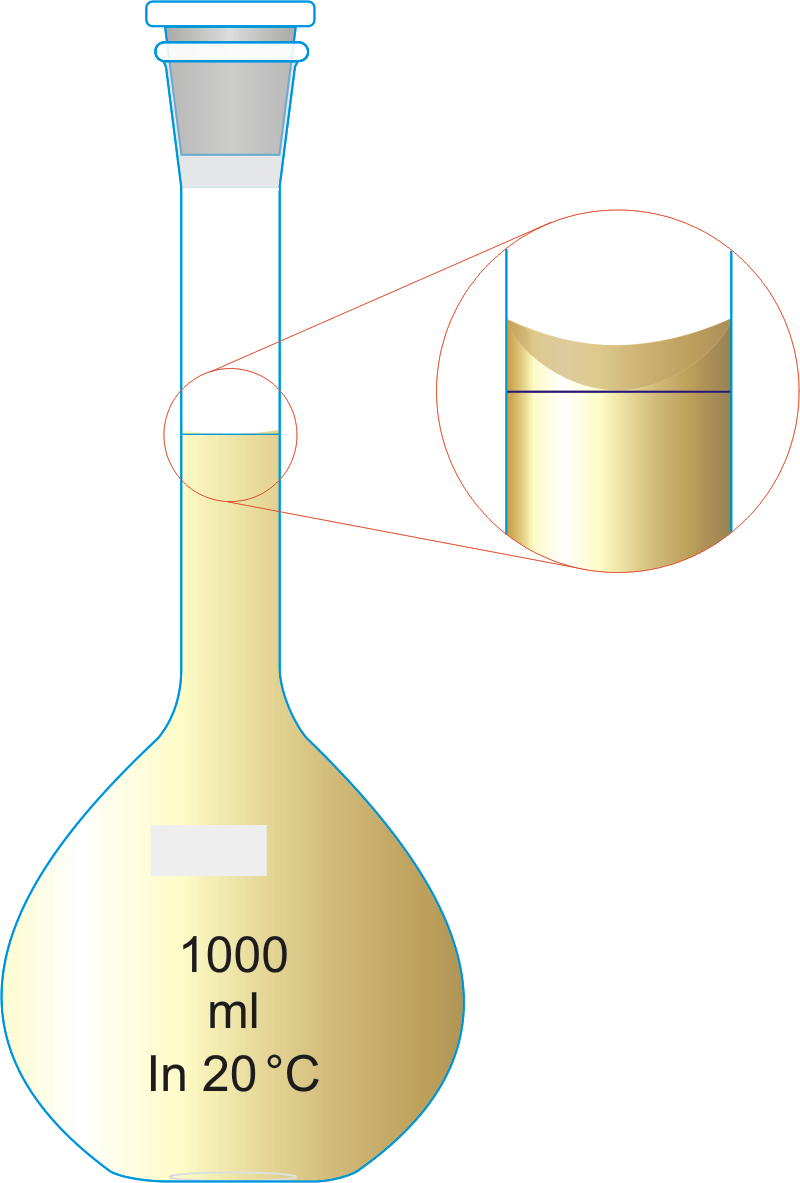 Solutions are made up by dissolving the quantity of solute (e.g. 10.0g of MgSO4) in a relatively small volume of solvent (e.g. water) in a volumetric flask and then 'making up to the mark' (e.g. 1000cm3) with further solvent to give a total volume of solution.
Solutions are made up by dissolving the quantity of solute (e.g. 10.0g of MgSO4) in a relatively small volume of solvent (e.g. water) in a volumetric flask and then 'making up to the mark' (e.g. 1000cm3) with further solvent to give a total volume of solution.
Note that if 10.0g of MgSO4 was added to 1000cm3 of water, then this would take the total volume of solution over 1000cm3 in this example, which is why we define concentration in terms of volume of solution rather than volume of solvent.
The volume of solvent or solution in any piece of volumetric glassware (e.g. volumetric flask, shown left) is always measured from the bottom of the meniscus (curved surface of a liquid).
In this example of 10.0g of MgSO4 in 1000cm3 of solution the concentration can be found:
Concentration (mol dm−3) = moles (mol) / volume of solution (dm3)
Moles = mass / molar mass = 10.0 / 104.38 = 0.0958037
Concentration = moles / volume = 0.0958037 / 1.000
Concentration = 9.58 × 10−2 mol dm−3 (3sf)
This could also be expressed in g dm−3, in which case it would be 10.0 g dm−3.