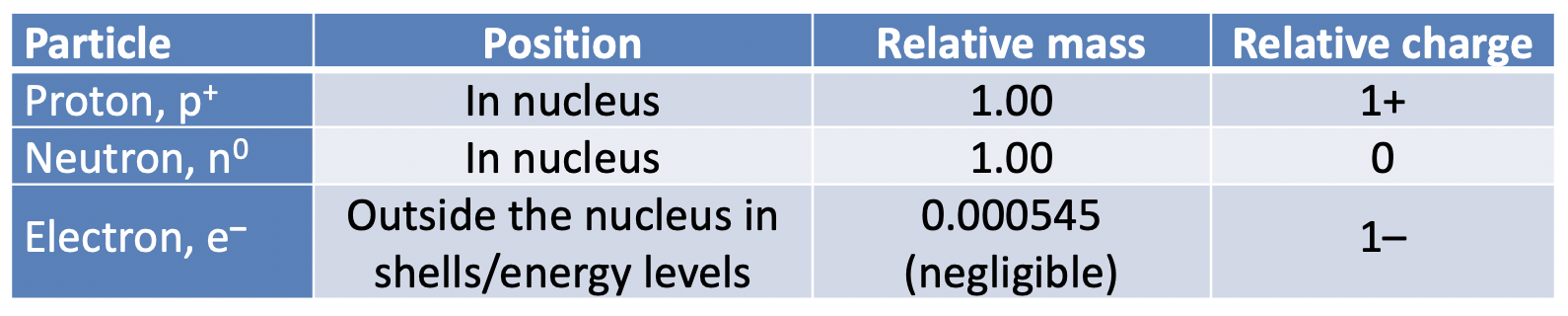
Atoms are the smallest unit of matter that have characteristic properties of chemical elements. Atoms are made up of three principal sub-atomic particles; protons, neutrons and electrons.
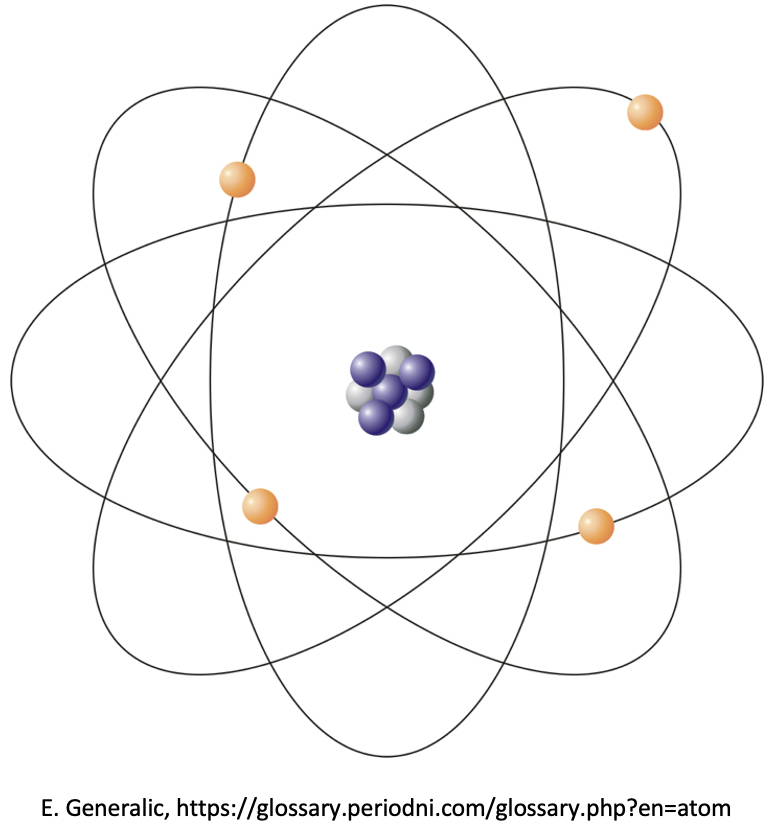 Protons and neutrons are found bound together by strong nuclear force in the nucleus of an atom. Protons and neutrons both have a relative mass of one unit (to three significant figures). The actual masses of the particles are very small indeed, which is why we use these relative masses. The masses are relative to one twelfth (1/12) of the mass of an atom of carbon-12. The nucleus of an atom therefore contains almost all the mass of the atom and is very dense.
Protons and neutrons are found bound together by strong nuclear force in the nucleus of an atom. Protons and neutrons both have a relative mass of one unit (to three significant figures). The actual masses of the particles are very small indeed, which is why we use these relative masses. The masses are relative to one twelfth (1/12) of the mass of an atom of carbon-12. The nucleus of an atom therefore contains almost all the mass of the atom and is very dense.
The relative mass of an electron is considered to be negligible (so small as to be insignificant). Electrons are found in the three-dimensional space outside the nucleus. There is a higher probability of finding particular electrons in certain places rather than others, and we call these places shells, energy levels and orbitals, depending on the model of the atom that we are using.
Protons carry a relative charge of 1+ and electrons carry a relative charge of 1−. The actual charges (measured in coulombs) on the particles are very small indeed, but are exactly equal but opposite, so it is easier for us to use relative charges. Neutrons carry no charge. All atoms are neutral overall because they have the same number of protons as electrons.